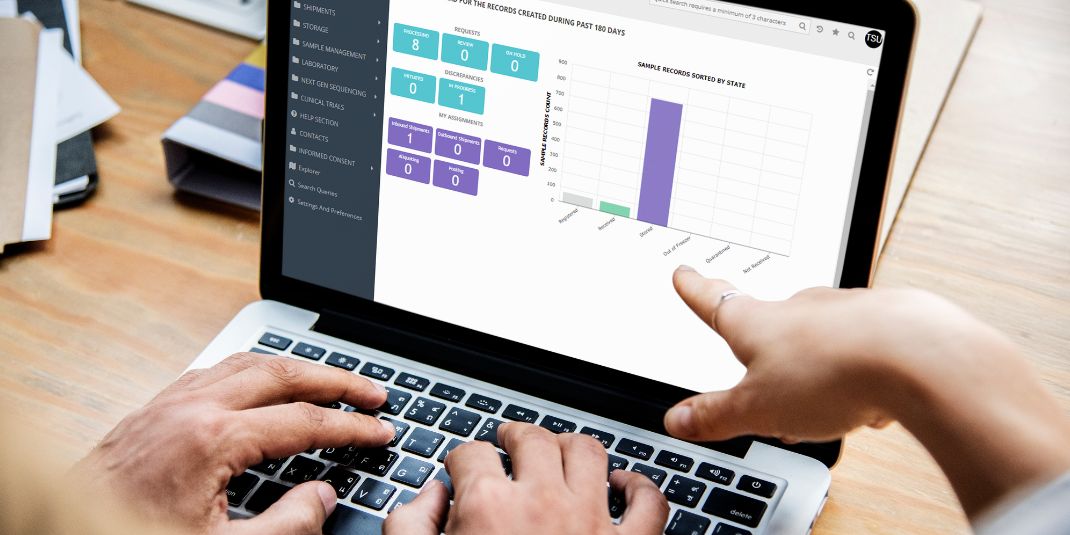
Taking a Fresh Look at Your Sample Inventory
Sample inventory management is a continuous process that supports the integrity of your data and research. Yet with samples and critical reagents added over time, sprawling inventories can strain valuable time, energy, and resources. An objective inventory review, conducted by better storage management experts, can help to optimize your sample and material storage and sustain […]
View Blog