Sample & Biological Material Storage
Comprehensive Storage Services for the Research Spectrum
From discovery to clinical trials and therapeutic products, Azenta Life Sciences provides comprehensive storage services that go above and beyond throughout the research spectrum. Our approach prioritizes risk mitigation, ensuring the secure storage, auditing, tracking, and delivery of your invaluable samples. Our quality management system attains global compliance and our subject matter experts collaborate to tailor lifecycle management solutions for your workflows.
Our internationally-certified staff and facilities handle logistics such as tracking, temperature-controlled packaging, and documentation. We adhere to over 150 industry best practices, setting the benchmark for excellence, so you can trust us to ensure that your irreplaceable biosamples are stored, audited, tracked, and delivered when you need them.
Want to request an expert consultation?
Key Features
Quality
Our quality management system is built to be compliant with the stringent requirements of GxP. Licenses, certifications and accreditations include ISO 9001, CAP, CLIA, NABP, EMA, PMDA and NRC.
Rapid Access
Rapid retrieval as fast as 24 hours for shipment of samples and materials across the globe.
Automation
Our own automated stores are available for additional layer of controlled cold chain management.
Inventory Management
24/7 visibility into inventory data, consolidation of third-party data, secure and compliant audit trail reporting, web-based retrieval and shipping on Limfinity® Sample Management System software to support 21 CFR part 11 compliance.
Business Continuity
Our biorepositories are equipped with multiple redundancy systems to ensure the highest sample and material integrity, even in the event of a natural disaster or other emergency.
Samples & Materials Stored

Human/Animal Biologics
blood, tissues, cells, DNA, RNA, plasma, forensics, and fluids

Biologic Test Materials
reagents, liquids, washes, and powders

Research Materials
slides, paraffin blocks, and powders

Agricultural Plant Banking
seeds, tissues, soil, sand (US only)
Storing APIs or Bulk Drug Substances?
Azenta offers a range of capabilities for your active pharmaceutical ingredients (APIs), bulk drug substances, and finished therapeutics, from storage and cold chain logistics management to distribution of drug products in their journey from development through commercialization.

Temperature Capabilities
State-of-the-art cGMP, GDP, and GTP-compliant short- and long-term storage ranging from ambient to -196°C.
- Ambient and Controlled Room Temperature (CRT)
- Freezer/Refrigeration (+5°C to -20°C)
- Ultra-low Freezer (-40°C to -80°C)
- Liquid Nitrogen (-150°C to -196°C)
Automated & Virtual Sample Management

Automated Storage
Our biorepositories benefit from the innovative automated storage systems you know and love from Azenta. These automated storage systems help minimize risk, facilitate more efficient workflows, and boost productivity.
- Automated flexible storage at a variety of temperatures
- Consistent and traceable sample handling including audit trail of all movements
- Automated retrieval of samples as part of our rapid access
- Increased sample integrity by minimizing exposure of surrounding samples during retrieval
Remote Inventory Management
Samples and materials stored with Azenta are managed within the Limfinity Sample Management System. This virtual sample management solution enables our team to efficiently manage your inventory and allows you to view all related data from a user-friendly customer portal. Through this informatics platform, you and your samples will benefit from:
- 21 CFR, part 11 compliance
- 24/7 visibility into inventory data
- Consolidation of third-party data
- Secure and compliant audit trail reporting
- Flexible tracking
- Web-based retrieval and shipping

Sample Management Solutions
Azenta Biorepository
Your samples can be stored at any of our sites, even multiple locations, and still leverage quick retrieval when you need to access them.
Customer-site
Our people, processes, and technology can be contracted to support sample management at your facility for quality storage.
Hybrid Options
Store your samples at both our site and yours for seamless sample management and consistent quality management.
Biorepository Locations
Our global state-of-the-art biorepository/biobanking solution facilitates your research with country specific or worldwide services. Our processes protect the identity, integrity, and chain-of-condition from the moment we receive the material until a retrieval is requested.
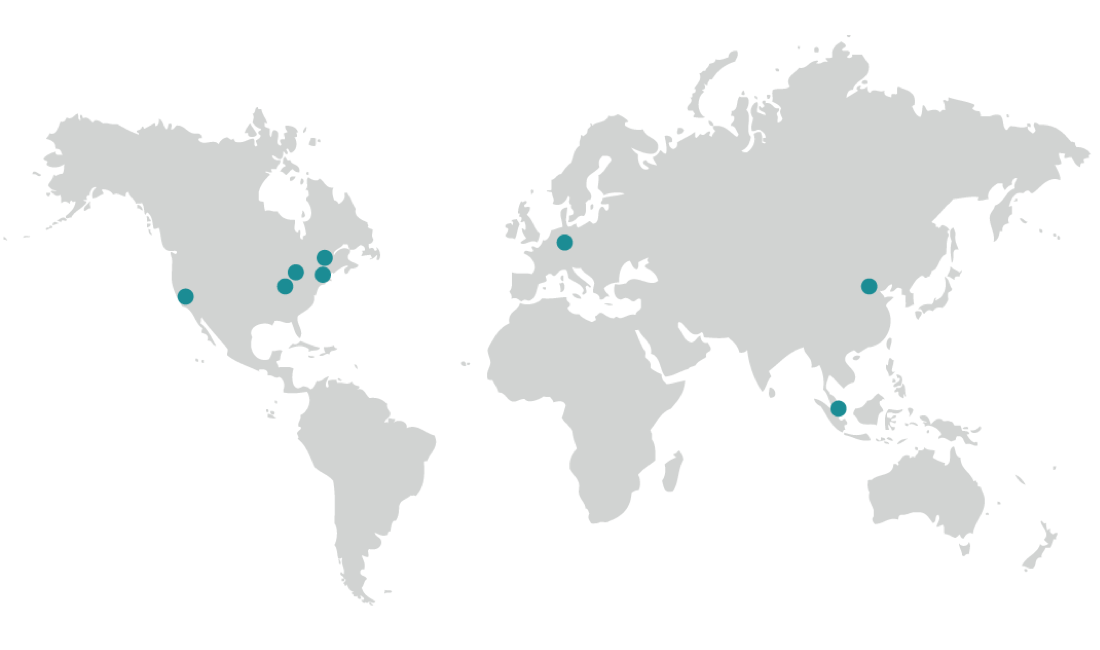
North America
Boston, MA, USA
Cleveland, OH, USA
Fresno, CA, USA
Indianapolis, IN, USA
Plainfield, IN, USA
Europe
Griesheim, Germany
Asia
Beijing, China
Singapore, Singapore
End-to-End Management

Project Management
Our Project Management Team works with each client to properly tailor project specifications. Your dedicated project management includes solutions and continuity plans customized according to your research goals, sample types, and project priorities.
Certified Logistics Management
Internationally-certified staff manage all logistics including tracking, temperature-controlled packaging, and documentation assistance. Our team is IATA, US DOT, and ADR certified.

Resources
Request an Expert Consultation
Want to learn more about these services? Speak to one of our experts by filling out and submitting the form.