Drug Products Storage & Distribution
Maintaining Secure Cold Chain Logistics, Every Step of the Way
Trust your manufactured drug products with the premier name in ultra-cold storage. Our capabilities range from storage and cold chain logistics management to pharmaceutical distribution of drug products in their journey from development through commercialization. Azenta Life Sciences understands the challenges that drug manufacturers like pharmaceutical companies and CDMOs face – starting with ultra-cold temperature environments. Manufactured drug products have rigorous cold storage standards, with specific needs, which are critical for the efficacy and shelf life of temperature-sensitive drugs. Adherence to these requirements can be very challenging, especially when the products need to be transported from/to various points-of-use
Our experts specialize in cGMP (Current Good Manufacturing Practice) controlled cold chain storage as well as cold chain management to help safeguard the integrity and quality of your indispensable products. From ambient temperature to our core competency in ultra-cold storage – we maintain secure cold chain logistics, every step of the way.
Want to request an expert consultation?
Key Features
Rapid Access
Rapid retrieval in as fast as 24 hours for shipment across the globe.
Cold Chain Logistics
End-to-end logistics including transport in our mobile biorepositories in the US to maintain chain of custody for bulk shipments.
Automation
Our own automated stores are available for additional layer of controlled cold chain management.
Inventory Management
24/7 access to material data through secure software that supports 21 CFR, part 11 compliance, providing real-time visibility to inventory and complete audit trail.
Quality
Quality management system (QMS) built to be compliant with the stringent requirements of GxP and 150+ unwavering best practices for regulated storage and logistics.
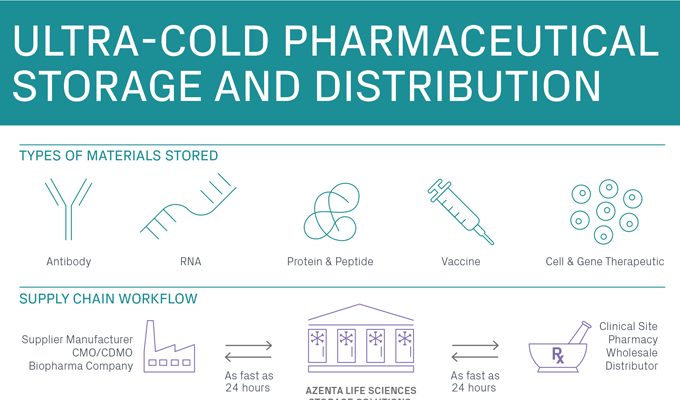
Materials Stored
Active Pharmaceutical Ingredients (APIs)
Bulk Drug Substances

Investigational Medicinal Products (IMPs)
Finished Drug Products
Retains

Temperature Capabilities
State-of-the-art cGMP, GDP, and GTP-compliant short- and long-term storage ranging from ambient to -196°C.
- Ambient and Controlled Room Temperature (CRT)
- Freezer/Refrigeration (+5°C to -20°C)
- Ultra-low Freezer (-40°C to -80°C)
- Liquid Nitrogen (-150°C to -196°C)
Leveraging Sample Management Expertise

Automation
Automated storage systems from Azenta allow flexible storage for a variety of labware and rapid access. These systems are available throughout our biorepositories to help minimize risk and support consistent and traceable handling of materials including an audit trail of all movements for an additional layer of visibility.
Inventory Management
All your materials stored with Azenta are managed within the Limfinity® Sample Management System which enables our team to efficiently manage your inventory and to always have visibility. Our software supports 21 CFR, part 11 compliance and provides secure audit trail reporting, flexible tracking, and web-based retrieval and shipping.

Gold Standard Storage & Distribution
- Accredited as a Drug Distributor by the NABP, Azenta holds licenses to distribute from our Indiana facility to all states and outlying territories of the United States
- PMDA Foreign GMP Manufacturer certified for GMP storage
- US State Board of Pharmacy
- GTP licensed by the USA FDA
- EMA inspected for GMP storage
- CAP Biorepository Accreditation Program
- Multiple redundancy systems and risk mitigation strategies with full backup ensuring preservation of the highest material integrity
Biorepository Locations
Our global state-of-the-art biorepository/biobanking solution facilitates your research with country-specific or worldwide services. Our processes protect the identity, integrity, and chain-of-condition from the moment we receive the material until a retrieval is requested.
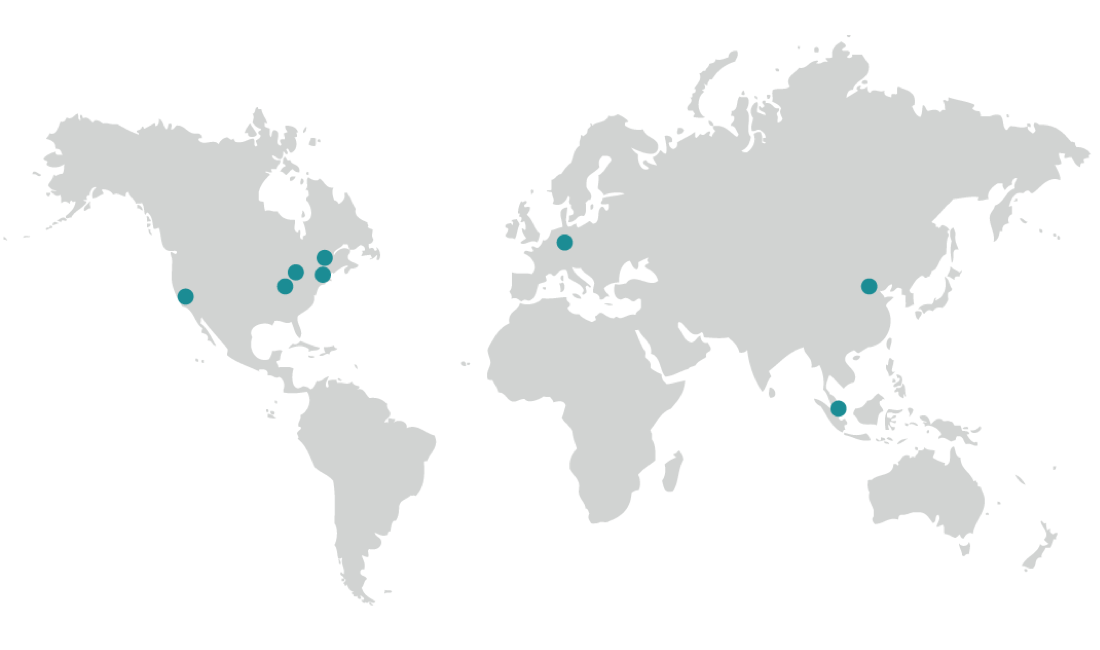
cGMP Storage and Distribution
Indianapolis, IN, USA
Griesheim, Germany
cGMP Storage
Boston, MA, USA
Cleveland, OH, USA
Fresno, CA, USA
Plainfield, IN, USA
Beijing, China
Singapore, Singapore
Customer Site Storage
Our people, processes, technology, and QMS can be contracted to support sample and material management at your facility.
Resources
Request an Expert Consultation
Want to learn more about these services? Speak to one of our experts by filling out and submitting the form.