
Advancing AI-Powered Sample Management: Automating Sample Registration through Imaging and Data Extraction
Publications & Posters
In this Poster
Streamline Workflows, Reduce Errors, Enhance Data Integrity, and Strengthen Compliance
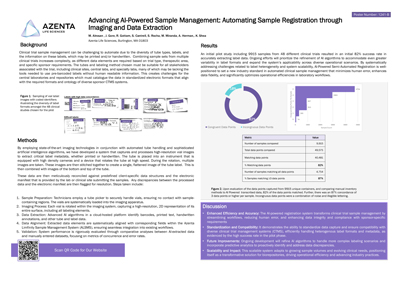
Clinical trial sample management can be challenging to automate due to the diversity of tube types, labels, and the information on these labels, which may be printed and/or handwritten. Combining sample sets from multiple clinical trials increases complexity, as different data elements are required based on trial type, therapeutic area, and specific sponsor requirements. The tubes and labeling method chosen must be suitable for all stakeholders associated with the trial, including clinical sites, central labs, and specialty labs, many of which may be lacking the tools needed to use pre-barcoded labels without human readable information.
Here, we demonstrate how imaging plus automated tube handling and AI algorithms can ease these challenges in central laboratories and repositories. By doing so, data can be cataloged in standardized electronic formats that align with the required formats and ontology of diverse sponsor clinical trial management systems (CTMS).
Read more about AI-powered clinical sample management!
Download Poster
Submit this form to access the poster!








