Clinical Trial Support
The benchmark in sample management™
Clinical sample management is a complex process. Clinical samples are often collected in many locations and then transported, stored, processed, and analyzed by multiple vendors. This fragmented approach to sample management can lead to spiraling sample storage costs, slow sample transfer times, data management difficulties, and poor chain of custody records. Azenta Life Sciences offers a complete, integrated sample management solution that solves these issues, enabling improved study timelines with increased sample integrity and sample utilization which, ultimately, can help accelerate trial outcomes and patient health.
Our extensive experience in managing clinical trials spans all therapeutic areas, regardless of phase or size, within one global, interconnected platform. Our experts will help support you throughout the process by customizing our suite of services to fit your individual sample management needs.
Want to request an expert consultation?
Key Features
Rapid Access
Retrieve samples and materials in as fast as 24 hours for shipment across the globe.
Inventory Management
Our proven end-to-end manual or automated sample management workflow provides 24/7 access to material data through secure software that supports 21 CFR, part 11 compliance, enabling real-time visibility to inventory and complete audit trail.
Quality
The quality management system (QMS) we use was built to support compliance with the stringent requirements of GxP and 150+ unwavering best practices for regulated storage and logistics including CLIA, CAP, FDA, ISO, and ISBER.
Flexibility
A range of integrated services can be customized to meet your sample management needs, from planning to discard, from an Azenta site or customer’s site.
Business Continuity
Our purpose-built redundancy system ensures the highest sample integrity, and our proven risk mitigation strategies keep your irreplaceable assets secure.
Clinical Sample Lifecycle Management
Plan
Our sample lifecycle solutions, protocols, and continuity plans are tailored to your research goals, sample types, and project priorities. Our project managers can help plan and manage any sample-related projects, including collection kit design, virtual sample inventory management, and aligning project-specific KPIs (Key performance indicators) and KQIs (Key quality indicators).
Additional support for your clinical trial management includes:
- Consent management
- Standard and custom labeling services
- Custom sorting and blinding services
Collect
Our cold-chain logistics services support active clinical trials, including consolidation of your sample assets into an efficient solution on-site or at an Azenta-site biorepository.
Transport
We guarantee an auditable chain of custody for each sample. Our experts partner with leading international surface and air carriers to securely ship samples across the globe, or our own transport experts are available for bulk shipments within North America.
Process
Our customizable solutions for sample preparation, analytical services, and data management meet the highest expectations of leading global organizations to maintain sample integrity and quality control.
Protect
Our carefully monitored and temperature-controlled environments, regulatory certifications (cGMP, GDP, GTP), secure access facilities, software that supports FDA compliance, and purpose-built redundancies account for every possible standard in protecting your sample and data assets.
Sample Access Options
- Active clinical trial samples: Our team ensures samples are retrieved, processed, stored, and distributed on time, every time. We can do much of your analysis at our GENEWIZ labs or send samples to central labs, specialty labs, or contract research organizations (CROs) as needed.
- Legacy samples for future use: Samples are kept easily accessible and with annotation, including consent.
- Retention samples: Samples are stored in secure units with audit trails for proper regulatory compliance.
Temperatures
- Ambient and controlled room temperature (CRT)
- Freezer/Refrigeration (+5°C to -20°C)
- Ultra-low freezer (-40°C to -80°C)
- Liquid nitrogen (-150°C to -196°C)
Retrieve
Our biorepository network guarantees sample retrieval and secure shipment within 24 hours of a request – from one vial to one thousand of your samples in a day, anywhere in the world.
Analyze
Sample value comes from the data contained within. Our single platform informatics and data integration solutions empower your discovery and development decisions. Whether you need a fit-for-purpose assay or to examine the multiome of a sample, leverage GENEWIZ Multiomics and Synthesis Services to gain further insights.
From kit to assay design to analysis, Azenta is your partner. Our services include:
- Biospecimen processing
- Genomics services
- Transcriptomic services
- Epigenomics services
- Proteomics services
Re-Purpose
Your sample’s journey doesn’t need to end here, sample utilization is extended by securing the highest integrity of preparation and bioprocessing based on best-practice protocols, ensuring your samples are ready to repeat the lifecycle for the next project.
Dispose
If you will no longer need a sample for your research, our compliant sample destruction processes provide an efficient way to manage the rationalization and disposal of stored samples.
Biorepository Locations
Our global network of interconnected biorepositories and sample bioprocessing laboratories accelerates clinical research timelines, reduces sample administration costs, and maximizes the value of your sample.
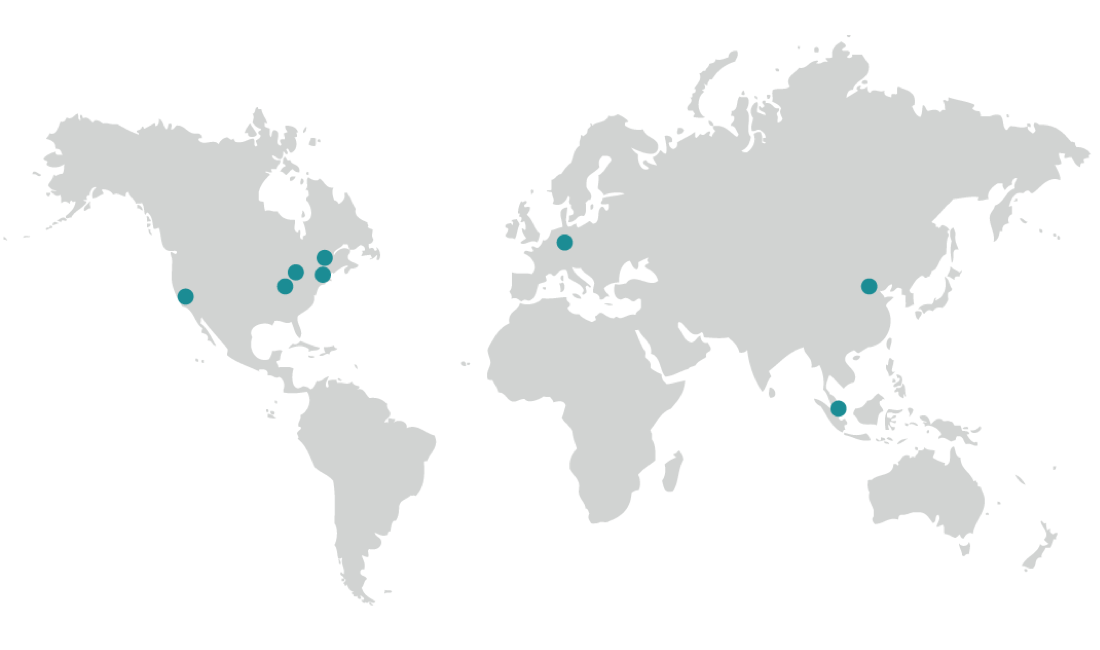
North America
Boston, MA, USA
Cleveland, OH, USA
Fresno, CA, USA
Indianapolis, IN, USA
Plainfield, IN, USA
Europe
Griesheim, Germany
Asia
Beijing, China
Singapore, Singapore
Resources
Request an Expert Consultation
Want to learn more about these services? Speak to one of our experts by filling out and submitting the form.