Monoclonal Antibody Production: Hybridoma vs. Recombinant

Antibodies are specialized proteins produced by the immune system that bind and neutralize foreign invaders such as viruses, bacteria, fungi, or parasites. Monoclonal antibodies (mAbs), composed of unique pairs of heavy and light chains, have been widely used by researchers to target antigens with high specificity. They have various applications in the diagnosis and treatment of a wide range of diseases including cancers, autoimmune disorders, and sexually transmitted infections. In recent years, technological improvements in antibody design have significantly expanded mAb development. Decreasing immunogenicity in humans, improving bioavailability, optimizing affinity and antigen-binding specificity, and other advances in protein engineering have contributed to better profiles for therapeutic antibodies.
The amount of mAb needed and the importance of factors such as cost, turnaround time, and regulatory compliance depends on the purpose. Hybridoma technology, which uses an animal-based approach, has long been the industry standard for monoclonal antibody production. However, increased commercial demands and quality requirements have caused many companies to explore recombinant antibody production as an alternative. To help you decide which method of antibody production is the most appropriate for your needs, we will look at the process, benefits, and challenges of each one.
Hybridoma antibody production
Advantages:
- Well-established and proven method
- Produces highly pure and specific antibodies
- Immortal source of monoclonal antibody
Disadvantages:
- Long generation time
- Animals required for discovery
- mAbs often require humanization
- Difficult and expensive to maintain in culture
- mAb yields can be highly variable
Hybridoma development is one of the more traditional methods used to generate monoclonal antibodies, enabling the production of highly sensitive binders. Hybridomas are cells formed via fusion between a short-lived antibody-producing B cell and an immortal myeloma cell. Each hybridoma constitutively expresses a large amount of one specific monoclonal antibody, and preferred cell lines can be cryopreserved for long-lasting mAb production.
Hybridoma generation is a five-step process that takes advantage of a host animal's natural ability to generate functional, highly specific, high-affinity antibodies (Figure 1).
- An immunogenic antigen is developed and optimized.
- A host animal is immunized with the antigen to elicit an immune response and initiate the process of B-cell maturation.
- These B cells are isolated from the spleen of the host animal and fused with tumor cells to generate hybridomas.
- The hybridomas are subjected to multiple rounds of screening and selection to identify ones that produce the best mAbs for the intended downstream application.
- The candidate cell lines are cultured, and monoclonal antibodies are purified from the supernatant. Alternatively, monoclonal antibodies can be produced in vivo by injecting hybridoma cells into the peritoneal cavity of an animal and harvesting antibodies from the resulting fluid (ascites).
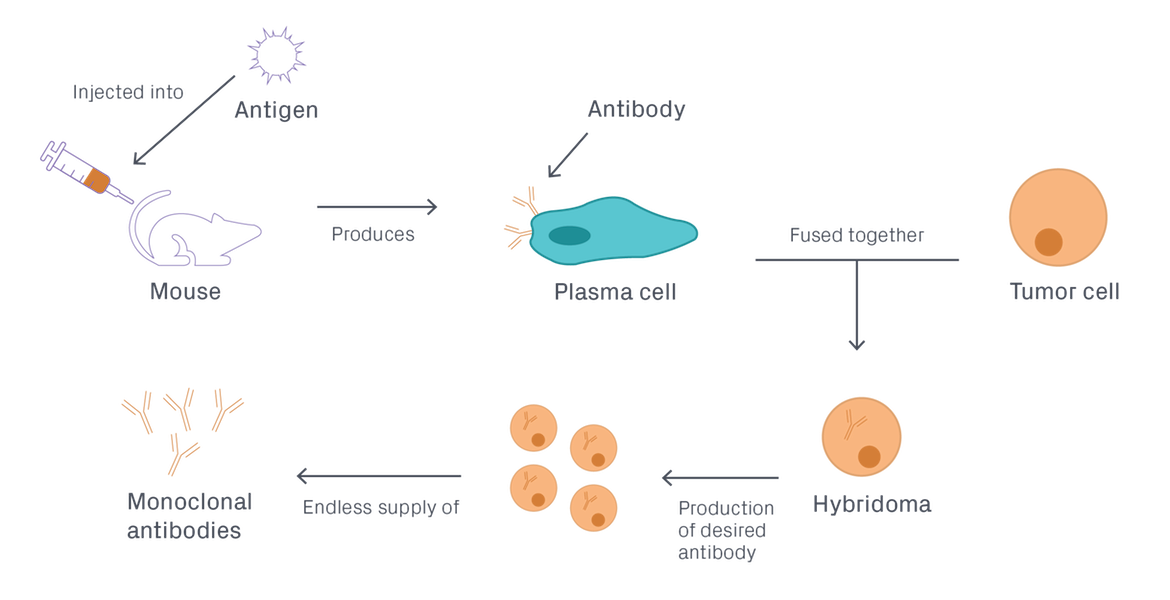
Figure 1. Hybridoma antibody production.
Although hybridoma antibody production is a well-established method of producing antibody, there are several drawbacks. More than 99% of the cells do not survive the fusion process, thus reducing the range of useful antibodies that can be produced against an antigen. Also, hybridomas are sometimes difficult and expensive to maintain in culture. If intended for therapeutic use, the antibody must undergo humanization, wherein parts of the protein sequence - usually the constant regions - are replaced with the human equivalent; otherwise, it may elicit an unintended immune response in humans. However, the main disadvantage with this technology is the difficulty of producing large quantities of stable human monoclonal antibodies, due to the absence of suitable myeloma cell lines.
Recombinant antibody production
Advantages:
- Increased reproducibility and control
- Decreased generation time
- Animal-free technology
- High-throughput production
Disadvantages:
- Requires technical expertise
A recombinant antibody is a synthetic antibody or antibody fragment that is generated by recombinant DNA technology. It is an in vitro alternative to the traditional hybridoma-based technology for creating monoclonal antibodies. Recombinant antibodies can be generated without animal immunization using a synthetic gene library and display technology. They have many applications and confer several advantages compared to traditional animal-derived antibodies due to their high specificity, sensitivity, and reproducibility.
A recombinant antibody approach typically involves the following steps (Figure 2):
- Generate an antibody library - a highly diverse collection of antibody DNA sequences in a common vector - from a natural or synthetic source.
- Use an in vitro system such as phage display to screen for antibodies that bind strongly to the antigen.
- Clone the antibody genes of interest into an expression vector.
- Express the protein in a host (bacteria, yeast, insect, or mammalian cell lines).
- Purify adequate amounts of functional antibody.
- Perform further testing to identify suitable candidates.
This strategy enables protein engineering and the ability to generate new antibody formats such as bispecific and antibody fragments (e.g., Fab, VHH, and scFv).
Figure 2. Recombinant antibody production.
The most prominent mammalian cell lines for recombinant mAb expression are CHO, NS0, Sp2/0, HEK293, and PER.C6. CHO cells are the workhorse for 70% of today’s industrially produced protein therapeutics, and as such, a valuable expression cell line during research and development. The selection of an expression system is determined by its ability to deliver high productivity with acceptable product quality attributes and the preferences of individual companies, which is often influenced by their historical experiences. While full-length antibodies require mammalian expression systems due to the occurrence of functionally and structurally important glycosylation, most antibody fragments and antibody-like molecules are non-glycosylated and can be more conveniently prepared in E. coli-based expression platforms. Expression in E.coli enables easier production with lower costs compared to other available expression platforms.
Recombinant antibodies have excellent batch-to-batch reproducibility because they have a known DNA and protein sequence and can be exactly replicated. Recombinant antibodies are also genetically stable and are not subject to issues observed in traditional hybridoma production such as genetic drift, antibody expression variation, and antibody sequence mutation that may cause non-specific binding.
Key Takeaways
- Hybridoma and recombinant DNA technologies are used to generate cell lines for monoclonal antibody production.
- While hybridoma technology is well established for reliable production of mAbs, its speed, quality, and throughput may not meet the needs of all applications.
- Recombinant antibody production provides a flexible, rapid platform for any mAb research or production environment.
Learn more about recombinant antibody production from gene synthesis through transient antibody expression and purification below.
References
1. Carvalho, L. S., da, O. B., de Almeida, G. C., de, J. D., & Parachin, N. S. (2017). Production Processes for Monoclonal Antibodies. In (Ed.), Fermentation Processes. IntechOpen. https://doi.org/10.5772/64263
2. National Research Council (US) Committee on Methods of Producing Monoclonal Antibodies. Monoclonal Antibody Production. Washington (DC): National Academies Press (US); 1999. 5, Large-Scale Production of Monoclonal Antibodies. Available from: https://www.ncbi.nlm.nih.gov/books/NBK100189/
3. Hybridoma technology: the preferred method for monoclonal antibody generation for in vivo applications. Samantha Zaroff and Grace Tan. BioTechniques 2019 67:3, 90-92
4. S. Stapleton, R. O' Kennedy, E. Tully,IMMUNOASSAYS | Production of Antibodies,Editor(s): Paul orsfold, Alan Townshend, Colin Poole, Encyclopedia of Analytical Science (Second Edition), Elsevier, 2005, Pages 306-316,
5. Alexander E. Karu, Christopher W. Bell, Tina E. Chin, Recombinant Antibody Technology, ILAR Journal, Volume 37, Issue 3, 1995, Pages 132–141, https://doi.org/10.1093/ilar.37.3.132
6. Kunert R, Reinhart D. Advances in recombinant antibody manufacturing. Appl Microbiol Biotechnol. 2016;100(8):3451-3461. doi:10.1007/s00253-016-7388-9
7. Sandomenico A, Sivaccumar JP, Ruvo M. Evolution of Escherichia coli Expression System in Producing Antibody Recombinant Fragments. Int J Mol Sci. 2020;21(17):6324. Published 2020 Aug 31. doi:10.3390/ijms21176324