Clearing Roadblocks in Early-Stage Cell & Gene Therapy Development
Under intense pressure to develop cell and gene therapies on tight timelines, biotech companies are encountering common bottlenecks for discovery and scale-up.
Therapies using adeno-associated virus (AAV) for gene delivery provide an interesting case study. AAV has many desirable properties as a therapeutic vector, but structural features of its genome can thwart traditional methods for molecular cloning and quality control.
We spoke with Dr. Conrad Leung, Fellow and Vice President of Technology Development at Azenta Life Sciences, to discuss these issues and recent innovations to address them.
What challenges are CGT companies facing during early development?
Many of our cell and gene therapy (CGT) customers are small biotech companies. They are in a unique spot compared to big pharma. For these small operations with a handful of employees, their biggest challenges are around how to scale things up. “How do I move it to the next level?”
Many of our customers work with us to scale up the things they need. They don’t have the staff to focus on this type of mundane work. Once they have the concept in mind—that is, they’ve designed the DNA —they outsource it to us to create the construct they need for their downstream applications. If you’re a cell or gene therapy company, you don’t want to invest in developing a vector—you want an expert to do that for you.
Why are some vectors for CGT particularly hard to work with?
Going back a few years, when we began providing AAV plasmids, I got a phone call from a customer saying they couldn’t sequence part of their construct called the inverted terminal repeat, or ITR. The DNA in an ITR region folds back on itself, forming a very strong hairpin structure, that inhibits a typical Sanger sequencing reaction.
To make matters worse, these regions are prone to acquiring spontaneous mutations—usually deletions—at various steps of the cloning workflow. Fully intact ITRs are important for AAV replication and packaging, so mutations can reduce the effectiveness of the therapy and increase variability in downstream results. With traditional analysis methods, scientists can’t keep close watch on these unstable regions.
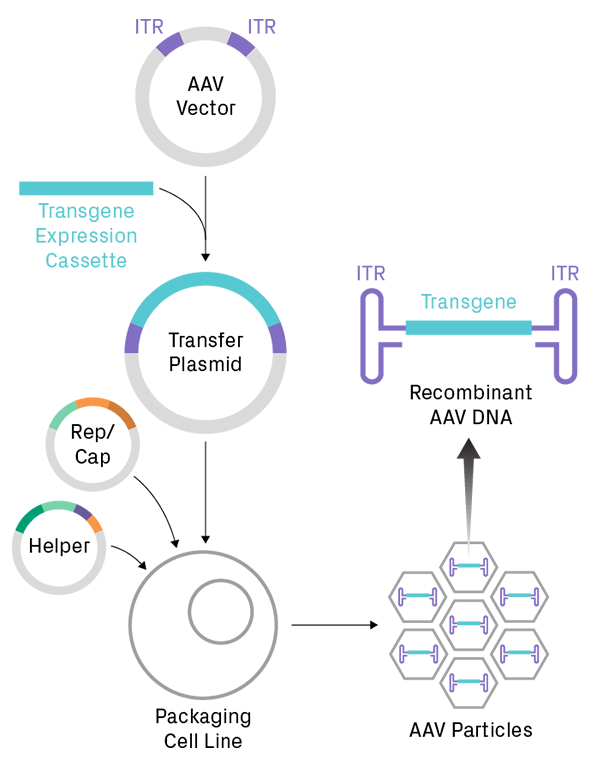
Production of AAV particles for therapeutic gene delivery. A gene of interest is cloned into an AAV vector to create a transfer plasmid, which is then introduced into a eukaryotic cell line. Generating functional viral particles requires co-transfection with several viral genes, supplied by packaging plasmids (i.e. Rep/Cap and helper plasmids). Recombinant single-stranded DNA, containing the transgene flanked by inverted terminal repeat (ITR) sequences, is packaged into capsids. The ITRs form very stable hairpins, which are problematic for amplification and sequencing of AAV plasmids during the early steps of the workflow.
How did you address the issues with ITR regions?
We developed a methodology to synthesize and prep AAV plasmids with higher fidelity along with a robust QC process to verify the accuracy of the construct. The game changer was a new Sanger sequencing protocol that could read the entire ITR region with a strong signal. It’s a powerful tool. You can now monitor ITR integrity with high confidence. If mutations do occur, we have an ITR correction process to fix them. These new tools alleviate a lot of headaches for researchers working with AAV, speeding up their overall workflow.
How do these new technologies for AAV help beyond the R&D stage?
As customers move closer to the pre-clinical and clinical phases, it becomes increasingly important to confirm the quality of their vectors. Contaminant DNA or mutated AAV sequences introduced during viral packaging can affect clinical safety and efficacy. As part of quality control, you need to measure the integrity and heterogeneity of the packaged material. As you move downstream and encounter more scrutiny from industry partners and regulatory bodies, sequencing becomes more important. Recently one of our customers reached out to us to help determine what percentage of viral purity is required for safety and efficacy. We’re currently evaluating commercial batches and working with the FDA to establish these guidelines.
Azenta Life Sciences provides a comprehensive range of solutions across every phase of CGT development. Learn how we can support the rigor of your program all the way from target discovery through scale-out.
About Conrad Leung

Conrad Leung, Ph.D., is a Fellow and Vice President of Technology Development at Azenta Life Sciences. He oversees a team of automation engineers and R&D scientists. His teams provide automation solutions to the company’s laboratories and develop innovative technology to support customers' scientific research. He has been with the company for more than 15 years.